Point-of-care bacteria detection system—integrating a bacteria test strip and a color analysis application
Introduction
Infectious diseases are still the primary cause for the heavy burden placed on health care and social medicine systems including health insurance (1). According to the World Health Organization, infectious diseases account for 54.4% of all deaths, mostly in countries with limited healthcare systems. In undeveloped or developing countries, such as Africa, South America, etc., infectious diseases are directly related to mortality (1,2), and are associated with regional food safety problems including water pollution, food hygiene, etc. (3).
Conventional methods of pathogen identification require growth and cultivation times, usually greater than 24 hours, before staining and observation (4-6). The microbial identification techniques widely used since the 19th century have been the standard, but it is common knowledge that bacterial growth conditions add significant time to the process (5,7). Recent developments in biotechnology have provided newer methods for pathogen detection (bacteria and virus detection), such as polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA). Although these methods have reduced the time required for detection (8-11), they must be carried out in a laboratory or clinical setting, and include expensive equipment and costs (12,13) that prohibit many patients from benefitting from these technological advances. For these reasons, detection in low-resource areas is still not improving.
The two currently available options for bacterial detection can be classified as (I) traditional bacterial culture, and (II) advanced biotechnology methods, respectively (8). The traditional bacterial culture method is to place a collected sample into a culture plate following appropriate dilution and then apply the spread-plate method. After placing the inoculated plate into an incubator for cultivation, colony counting based on sample dilution is used to determine a bacterial calculation, i.e., the colony forming unit (CFU), for the sample (14), thus determining the bacterial concentration and severity of sample infection status. The disadvantages of this method are that it is limited to the requirements of sample transfer, transportation, laboratory equipment, incubation time, and clinical expertise. Because the process can take up to 2 days, response and treatment is delayed and the lack of timely care may exacerbate the situation. Biotechnology methods primarily focus on the use of PCR and ELISA. Although these methods are more time efficient, mitigating factors associated with the test subject and the technological complexity still delays results for several hours at best. More importantly, such techniques are expensive and require experienced professionals to implement. For these reasons, we undertook the development of a real-time bacterial detection technique that could be inexpensive (less than 1 USD), easy to operate (low technical threshold), portable (only requires a test strip and a smartphone), and rapid (15–20 minutes total elapsed time).
Methods
Bacteria test strip development
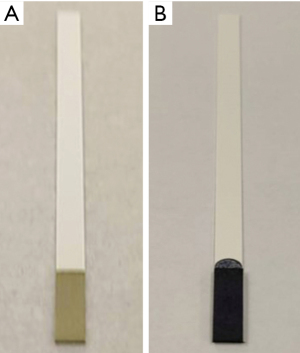
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) can interact with mitochondria in living cells. Viable cells contain succinate dehydrogenase, which reduces MTT to its intensely purple formazan form. Phenazine methosulfate (PMS) is an intermediate electron acceptor that facilitates this reaction. We used Whatman Fusion Paper 5TM (Sigma-Aldrich, Burlington, Massachusetts, USA) to absorb both MTT and PMS, then attached the impregnated paper as a thin section on our completed test strip (Figure 1).
Bacteria culture
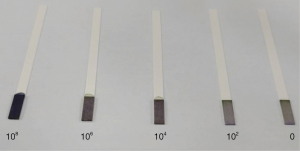
The bacteria we used in this experiment was common Escherichia coli. We cultivated our bacteria using tryptic soy broth culture medium purchased from Sigma Aldrich. After adding bacteria and culture medium into a culture tube, we incubated the samples for 24 to 48 hours. We used the Microvolume Spectrophotometer (Nanodrop, Thermo Scientific, Waltham, Massachusetts, USA) to test the concentration of bacteria, making serial dilutions of 108, 106, 104, 102, 0 CFU/mL as standards for subsequent experiments.
Bacteria test strip testing procedure
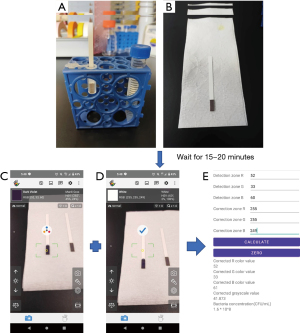
We placed each of the different concentration standards in the middle of the detection zone of our test strip, waited for the strip reaction to take place, and then for the strip to completely dry, which took approximately 15–20 minutes. The resulting color changes from each bacteria concentration are shown in Figure 2.
RBG color analysis
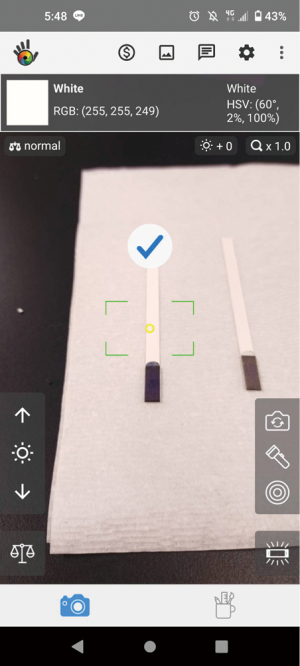
When the test strip reacts with the sample, the resulting color is visible, but the color intensity is difficult to determine using the naked eye. The test strips currently on the market are not quantifying, they are qualifying only, i.e., they only determine whether or not the detection substance is present in the sample. Here, we sought to analyze test strip color intensity using a smartphone to provide greater accuracy. Using a freely available smartphone application (APP) called Color grab (available on Google Play, developed by Loomatix, Matam, Haifa, Israel; Figure 3) and aligning the test strip with the smartphone camera, the post-reaction RGB color value of the test strip can be obtained for bacteria concentration determination.
Color analysis and concentration conversion APP
In order to distinguish color intensity there are still some hurdles to overcome in order to calculate RGB value: (I) differences in camera quality and characteristics; (II) shooting environment; (III) differences in standard RGB values.
We used the following methods to solve these issues and develop the first version of our APP.
(I) We used the upper white area of the strip as our correction standard zone, analyzed its RGB value, and compare that value with the RGB value in the detection zone. We collected both visual samples under the same smartphone lens conditions, and used the ratio between the detection zone and the correction standard zone to calculate and resolve issues associated with smartphone camera differences.
(II) The RGB value of the correction area obtained above corresponds to the color change on the test strip. Since the environment would influence both detection and correction zones equally, we used the ratio of the correction standard zone and the environment to adjust the detection zone. The environmental error was removed from the two RGB values using the following formula:
The environmentally corrected RGB value was then used to determine a standardized color intensity change.
(III) To determine color intensity change, since the three types of RGB values did not change in proportion, we converted the RGB values to grayscale using standard grayscale conversion formula. This aided in unifying the standard across RGB value interpretations. Following conversion, we could then apply the following formula to determine a standardized color intensity value change:
The resulting value resolved any concerns regarding variable color values for multiple colors.
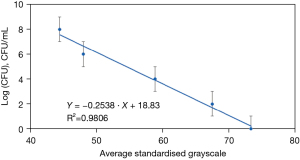
By making multiple measurements using the serial bacterial solution concentrations on our test strip, we could determine the relationship between grayscale value and bacterial solution concentration (Figure 4).
We then leveraged the developed calibration curve formula obtained in Figure 4 in our self-developed APP (X for grayscale, Y for bacterial solution concentration):
For user-friendliness, we included all the formulas and calculations used {Eq. [1]–[5]} into our APP. This facilitates the input of RGB value and the consequent result, i.e., bacterial concentration, as determined by our test strip.
Results
The data obtained through the above experiment was integrated and written into the user APP, and the above experiment was repeated several times. The use of this test strip with the self-developed APP can achieve a verifiable rate of accuracy. As summarized in Figure 5, for example, the observed sample concentration of 1.6×108 CFU/mL is quite close to the applied concentration of 108 CFU/mL.
Discussion
At present, it is possible to convert the color change displayed by the test strip into bacterial concentration, thus providing quantified information. Moving forward, the camera RGB interpretation APP (Color grab) may be combined with the analysis APP we manufacture to further enhance user convenience.
Conclusions
The development of this test strip can provide significant time, cost, and urgency of treatment advantages. Used in remote settings or clinics, it can greatly shorten the time required for detection (about 15–20 minutes) and does not require expensive equipment of technical expertise. The use of the test strip is also relatively simple, and completing the data calculation via the APP is very user-friendly as long as there is a program link. From a cost perspective, this approach is exceptional. The manufacturing cost of a single test strip is less than 1 USD. For medical resource-poor areas such as developing or undeveloped countries, this kind of testing is highly practical and impactful. Test strips of this nature may be used to test for urinary tract infections, sepsis, water pollution and others, bringing much-needed and impactful health care for a broad and under-served population, while reducing the already excessive burden on health care systems.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://ht.amegroups.com/article/view/10.21037/ht-23-1/dss
Peer Review File: Available at https://ht.amegroups.com/article/view/10.21037/ht-23-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ht.amegroups.com/article/view/10.21037/ht-23-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
- Mitsakakis K, D'Acremont V, Hin S, et al. Diagnostic tools for tackling febrile illness and enhancing patient management. Microelectron Eng 2018;201:26-59. [Crossref] [PubMed]
- Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis 2002;2:103-10. [Crossref] [PubMed]
- Lagier JC, Edouard S, Pagnier I, et al. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev 2015;28:208-36. [Crossref] [PubMed]
- Reali S, Najib EY, Treuerné Balázs KE, et al. Novel diagnostics for point-of-care bacterial detection and identification. RSC Adv 2019;9:21486-97. [Crossref] [PubMed]
- Puttaswamy S, Lee BD, Sengupta S. Novel electrical method for early detection of viable bacteria in blood cultures. J Clin Microbiol 2011;49:2286-9. [Crossref] [PubMed]
- Vila J, Gómez MD, Salavert M, et al. Methods of rapid diagnosis in clinical microbiology: Clinical needs. Enferm Infecc Microbiol Clin 2017;35:41-6. [Crossref] [PubMed]
- Liao YH, Muthuramalingam K, Tung KH, et al. Portable Device for Quick Detection of Viable Bacteria in Water. Micromachines (Basel) 2020;11:1079. [Crossref] [PubMed]
- Carbonnelle E, Mesquita C, Bille E, et al. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem 2011;44:104-9. [Crossref] [PubMed]
- Itoh S, Kariya M, Nagano K, et al. New rapid enzyme-linked immunosorbent assay to detect antibodies against bacterial surface antigens using filtration plates. Biol Pharm Bull 2002;25:986-90. [Crossref] [PubMed]
- Järvinen AK, Laakso S, Piiparinen P, et al. Rapid identification of bacterial pathogens using a PCR- and microarray-based assay. BMC Microbiol 2009;9:161. [Crossref] [PubMed]
- Lazcka O, Del Campo FJ, Muñoz FX. Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron 2007;22:1205-17. [Crossref] [PubMed]
- Shih CM, Chang CL, Hsu MY, et al. Paper-based ELISA to rapidly detect Escherichia coli. Talanta 2015;145:2-5. [Crossref] [PubMed]
- Rompré A, Servais P, Baudart J, et al. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods 2002;49:31-54. [Crossref] [PubMed]
Cite this article as: Lin YC, Pan SC, Cheng CM. Point-of-care bacteria detection system—integrating a bacteria test strip and a color analysis application. Health Technol 2023;7:1.