
Correlation between the ownership of Coronavirus disease 2019 (COVID-19) vaccine patents and the choice of a patent waiver: cases in the US, the UK, Germany, and India
Introduction
Coronavirus disease
Coronaviruses are RNA viruses that appear spherical with crown-like spikes on the surface under an electron microscope. They can be classified into four subgroups: alpha, beta, gamma, and delta coronaviruses. Moreover, seven pathogenic human coronaviruses (HCoVs) have been identified; these include two alpha coronaviruses (HCov-229E and HCoV-NL63), two beta coronaviruses (HCov-HKU1 and HCov-OC43), Middle East respiratory syndrome-related coronavirus (MERS-CoV), severe acute respiratory syndrome-related coronavirus (SARS-CoV), and the newly identified SARS-CoV-2 (1). The mode of transmission of the virus is from the saliva of an infected person (2).
Statistics about coronavirus disease cases
According to the data on the Worldometer website on January 21, 2022, the number of coronavirus disease 2019 (COVID-19) cases was 343,549,356, with the number of deaths and recovered cases being 5,595,233 and 275,132,256, respectively. The United States (US) had the highest number of cumulative cases (70,544,862 cases). The total deaths and recovered cases in the US were 883,903 and 44,047,799, respectively. It was followed by India, with a total of 38,566,027 cases. The total deaths and recovered cases in India were 488,422 and 36,058,806, respectively. Brazil was at the third position, with a total of 23,588,921 cases. The total deaths and recovered cases in Brazil were 622,251 and 21,851,922, respectively. Taiwan was at the 165th position, with a total of 18,109 cases. The total deaths and recovered cases in Taiwan were 851 and 16,199, respectively (3).
Types of vaccines
Messenger RNA (mRNA) vaccine
mRNA vaccines use the genetic engineering technique. In these vaccines, mRNA instructs cells for producing the spike (S) protein that can be found on the surface of the COVID-19 virus. After vaccination, muscle cells can generate S protein pieces and present them on the cell surface, thereby helping the body to produce antibodies. When a vaccinated individual is later infected with the COVID-19 virus, these antibodies can defend against the virus. The Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) COVID-19 vaccines use this technique.
Vector vaccine
In vector vaccines, a strand of DNA encoding the COVID-19 S protein is introduced into a different virus (viral vector). This type of vaccine can deliver the genetic material and instruct cells to prepare copies of the S protein. As cells present S proteins on their surface, the immune system gets stronger by producing antibodies and white blood cells. When a vaccinated individual is later infected with the COVID-19 virus, these antibodies can defend against the virus. The Janssen (Johnson & Johnson, Titusville, New Jersey, USA) (Ad26.COV2.S) and AstraZeneca/University of Oxford (AZD1222) vaccines use this technique.
Protein subunit vaccine
In subunit vaccines, the genetic recombination technique is used to prepare harmless S proteins. This stimulates the immune system to produce antibodies. When a vaccinated individual is later infected with the COVID-19 virus, these antibodies can defend against the virus.
The Novavax (NVX-CoV2373) vaccine was developed using this technique (4).
Inactivated vaccine
The technique for producing inactivated vaccines is more traditional than those mentioned above. Inactivated vaccines consist of a weakened (or attenuated) virus in which the genetic material is destroyed. Such a virus can still replicate to stimulate the immune system without causing illness (5). The Sinovac (Ad26.COV2.S, Haidian, Beijing, China) COVID-19 vaccine uses this technique.
Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS)
The Agreement on TRIPS took effect on 1st January, 1995. It covers copyright and related intellectual property rights. This agreement requires member countries to provide patent protection in the field of pharmaceuticals (6). TRIPS has had a huge impact on the global pharmaceutical industry (7). On the one hand, considering the spread of the COVID-19 pandemic, the results of pharmaceutical research are crucial for maintaining public health and safety. On the other hand, patents that grant innovators monopolistic rights over their knowledge are important for the pharmaceutical industry in order to secure profits. This has triggered international discussions on whether to exempt vaccine patents for a certain period in order to accelerate global cooperation for fighting the pandemic.
Research question
According to the rules of TRIPS, member states are obliged to adhere to patent legal protection in order to encourage innovations. Nonetheless, member states with a higher level of technologies would benefit more from the legal rules, while states with lesser technological advances may be blocked from accessing the vaccines because of patent protection. The research question for this article is to assess the correlation between the number of patents and the choice of a patent waiver by comparing the cases in the US, the United Kingdom (UK), Germany, and India.
Methods
Documentary analysis
The authors collected various documents, including relevant literature, official statements from governments and pharmaceutical companies, and conference records of the World Trade Organization (WTO), to analyze the research question.
Patent search
The authors adopted the Global Patent Search System (GPSS) to perform a patent search. The patent search results provided in Table 1 are dated October 4th, 2021. The search consisted of names of pharmaceutical companies and their respective vaccine technologies as keywords. The terms “mRNA” and “vector” were also used as keywords to perform the patent search. The search results were further filtered by patent status. Patents shown in both patent pre-grant publication and patent grant columns were considered under a single patent grant column only.
Table 1
| Patentee (biotechnology company) | Countries | Vaccine | Technology | Patent numbers | Search query |
|---|---|---|---|---|---|
| Pfizer | US | BNT162b2 | mRNA | 494 | Pfizer AND VACCINE AND mRNA |
| BioNTech | Germany | BNT162b2 | mRNA | 322 | BioNTech AND VACCINE AND mRNA |
| Moderna | US | mRNA-1273 | mRNA | 220 | Moderna AND VACCINE AND mRNA |
| AZ | UK | AZD1222 | vector | 42 | AZ AND VACCINE AND vector |
| Gennova | India | HGC019 | mRNA | 2 | Gennova AND VACCINE AND mRNA |
US, United States; UK, United Kingdom.
Results
Patent search
The data shown in Figure 1 are dated on 6th of January, 2022. The terms “vaccine” and “mRNA” were used as keywords for the search, generating 112,740 initial results and 91,972 results when filtered by patent status. Figure 1 shows the top 20 countries/regions with mRNA vaccine patentees.
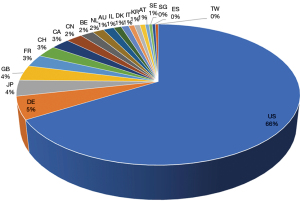
The search results shown in Figure 2 are limited from 1st of January, 2005 to 6th of January, 2022. The terms “vaccine” and “vector” were as keywords for the search, generating 122,103 initial results and 97,403 results when filtered by patent status. Figure 2 shows the top 20 countries/regions with vector vaccine patentees.
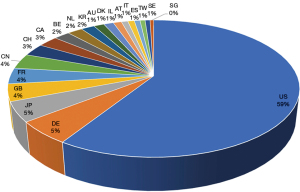
Patent waiver
India
On 2nd October, 2020, India and South Africa made a joint submission (IP/C/W/669) to the WTO for the suspension of some intellectual property protections under TRIPS in relation to the containment, prevention, and treatment of COVID-19 (hereafter referred to as the patent waiver proposal) (8).
This patent waiver proposal emphasizes that the COVID-19 outbreak has caused a sharp increase in the global demand for medical products. Consequently, many countries have faced shortages, stopping these countries from effectively containing the outbreak. The abrupt shortages of medical products have also put people’s lives at risk and resulted in many avoidable deaths. Furthermore, without a sufficient and quick supply of necessary medical products, the world would inevitably continue to suffer from the COVID-19 pandemic.
Therefore, it is imperative that intellectual property rights do not act as obstacles that would hinder or potentially hinder the timely supply of affordable medical products to patients.
In light of these circumstances, India and South Africa proposed that the TRIPS Council provides recommendations as early as possible to the General Council regarding a waiver from the implementation, application, and enforcement of Sections 1, 4, 5, and 7 of Part II of TRIPS in relation to the prevention, containment, and treatment of COVID-19.
While the Indian government took the lead in the international arena to argue for a patent waiver, the Indian company Gennova Biopharmaceuticals (Pune, Maharashtra, India) developed the vaccine HGC019 with the American company HDT Bio Corp. (Seattle, Washington, DC, USA) (9). However, Gennova Biopharmaceuticals owns only two patents in this field.
US
Although the US was strongly against suspending the intellectual property protections at the WTO, according to a report in the New York Times dated May 5th, 2021, President Biden had come under increasing pressure to support the proposal drafted by India and South Africa (10).
Katherine Tai, the US Trade Representative stated, “This is a global health crisis, and the extraordinary circumstances of the COVID-19 pandemic call for extraordinary measures. The administration believes strongly in intellectual property protections, but in service of ending this pandemic, supports the waiver of those protections for COVID-19 vaccines”. She suggested that this decision is key to end this global pandemic (10).
While President Biden declared his support for the patent waiver proposal, it was not surprising that the Pharmaceutical Research and Manufacturers of America (also known as PhRMA, Washington, DC, USA), which consists of major pharmaceutical companies, including COVID-19 vaccine makers, launched a campaign against his decision (11).
UK
After the US President Biden expressed his support for the patent waiver proposal, the UK government started talks about a plan to waive COVID-19 vaccine patents in order to boost the production of vaccines in low- and middle-income countries (12).
The discussions were ongoing amid increasing pressure from the society, asking the government to follow the US in supporting the patent waiver proposal.
In May 2021, more than 400 supporters of the patent waiver proposal across various sectors came forward and called on the UK government to follow the US in supporting the patent waiver plan for COVID-19 vaccines (13).
In December 2021, several members of the UK Parliament jointly signed a motion stating the following: “This House expresses its concern at the UK Government’s failure to adequately support low income countries in the fight against coronavirus; notes that 81% of the eligible UK population has received two doses of a COVID-19 vaccine, compared to less than 8% of people across Africa according to Amnesty International; further notes that the UK government are blocking countries from producing their own vaccines by refusing to support the temporary waiver on vaccine patents which is supported by the South African and Indian governments…” (14).
Although the UK government is under pressure from the society and parliamentary scrutiny, so far, there is no sign that it would support the patent waiver proposal.
Germany
The German government decided not to back the US President’s support for the patent waiver proposal for COVID-19 vaccines in May, emphasizing that the main solution is not to suspend intellectual property protections but to increase the capacity and ensure the quality of medical products (15).
The German government insisted that the main obstacles in the way of massive vaccine production are capacity and quality standards instead of patents. “The protection of intellectual property is a source of innovation and must remain so in the future”, said the spokeswoman in a statement. Germany’s opposition to this proposal remains strong to this date and is deemed to have a significant influence on the European Union (EU) position (16).
Discussion
As per the results of the patent search, the top 5 countries where mRNA vaccine patentees are based are the US, Germany, Japan, the UK, and France. The order is the same in the case of vector vaccines. The US has the predominant position in mRNA and vector vaccine technologies. In this article, examples from the US, Germany, and the UK are used to discuss their choice of a patent waiver because these countries not only are among the top 5 countries with vaccine patentees but also have developed their own vaccines. Germany and the UK clearly refused to back the US in supporting India’s patent waiver proposal. Although the US government once expressed support for this proposal against big pharmaceutical companies, it declined to support the patent waiver proposal at the WTO in September 2021.
In conclusion, the patent waiver proposal is supported by lesser developed countries that are not the top patent owners of vaccines. Countries that are the top patent owners of vaccines, or the biggest beneficiaries of patent protections, are either against the proposal or responding very slowly.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ht.amegroups.com/article/view/10.21037/ht-22-5/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taiwan Centers for Disease Control. Coronavirus disease 2019. Last Accessed 1-21-2021. Available online: https://www.cdc.gov.tw/En/Category/MPage/Zw2wYxRiPGMiZPhXlB-pmw
- WHO. Coronavirus disease (COVID-19). Last Accessed 10-6-2021. Available online: Https://Www.Who.Int/Health-Topics/Coronavirus#Tab=Tab_1
- Worldmeter. COVID-19 coronavirus pandemic. Last Accessed 1-21-2022. Available online: https://www.worldometers.info/coronavirus/
- Mayo Clinic. Different types of COVID-19 vaccines: how they work. Last Accessed 10-6-2021. Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-covid-19-vaccines/art-20506465
- Gavi. There are four types of COVID-19 vaccines: here’s how they work. Last Accessed 10-6-2021. Available online: https://www.gavi.org/vaccineswork/there-are-four-types-covid-19-vaccines-heres-how-they-work
- World Trade Organization. Overview: the TRIPS agreement. Last Accessed 1-21-2022. Available online: https://www.wto.org/english/tratop_e/trips_e/intel2_e.htm
- Lin TY. Issues and resolutions in pharmaceutical patents and public health under the WTO TRIPS agreement. Chengchi Law Rev 2004;78:267-342.
- WTO. Waiver from certain provisions of the trips agreement for the prevention, containment and treatment of COVID-19. Last Accessed 3-4-2022. Available online: https://Docs.Wto.Org/Dol2fe/Pages/Ss/Directdoc.Aspx?Filename=Q:/Ip/C/W669.Pdf&Open=True
- NEWS18. Explained: why India-made Gennova Vax could give protection at a fraction of cost of other mRNA shots. Last Accessed 10-6-2021. Available online: https://www.news18.com/news/explainers/explained-all-you-need-to-know-about-indias-first-homegrown-rna-vaccine-3961247.html
- Kaplan T, Sheryl Gay Stolberg SG, Rebecca Robbins R. Taking ‘extraordinary measures,’ Biden backs suspending patents on vaccines. Last Accessed 5-5-2021. Available online: https://www.nytimes.com/2021/05/05/us/politics/biden-covid-vaccine-patents.html
- Schwartz B. Big pharma lobbyists launch campaign against Biden over Covid vaccine patent waiver. Last Accessed 6-1-2021. Available online: https://www.cnbc.com/2021/06/01/big-pharma-launches-campaign-against-biden-over-covid-vaccine-patent-waiver.html
- Summers H. Britain in talks to waive Covid vaccine patents to improve global access to jabs. Last Accessed 5-20-2021. Available online: https://www.theguardian.com/global-development/2021/may/20/britain-in-talks-to-waive-covid-vaccine-patents-to-improve-global-access-to-jabs
- Kelly E. Call for UK to back patent waiver on COVID-19 vaccines. Last Accessed 5-11-2021. Available online: https://sciencebusiness.net/news/call-uk-back-patent-waiver-covid-19-vaccines
- WTO. Covid-19 vaccination patent waiver recommendation to the World Trade Organisation EDM (Early Day Motion)779. Last Accessed 12-15-2021. Available online: https://edm.parliament.uk/early-day-motion/59281/covid19-vaccination-patent-waiver-recommendation-to-the-world-trade-organisation
- Reuters. Germany rejects U.S. proposal to waive patents on COVID-19 vaccines. Last Accessed 5-6-2021. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/germany-opposes-us-plan-waive-patents-covid-19-vaccines-2021-05-06/
- Green A. How Germany's new coalition could change the fate of the TRIPs waiver. Last Accessed 11-22-2021. Available online: https://www.devex.com/news/how-germany-s-new-coalition-could-change-the-fate-of-the-trips-waiver-102159
Cite this article as: Wang T, Chiang Y. Correlation between the ownership of Coronavirus disease 2019 (COVID-19) vaccine patents and the choice of a patent waiver: cases in the US, the UK, Germany, and India. Health Technol 2022;6:4.


