
An intraabdominal stent system for gasless laparoscopic surgery in animal model: potential benefits and limitations
Introduction
Laparoscopic surgery had been developed for decades. Conventionally, the most commonly used method to create a suitable operative filed is artificial pneumoperitoneum using carbon dioxide (CO2). Inflation of CO2 can expand the peritoneal cavity, with which a proper operative filed can be created. Because CO2 is a chemically inert gas and can be easily absorbed by the human body, using CO2 to achieve artificial pneumoperitoneum had been a routine in current practice of laparoscopic surgery.
Inflation of CO2 is usually controlled by an external machine which can regulate the rate of inflation and the pressure in the abdominal cavity. When we use higher pressure of inflation, there would be more CO2 in the peritoneal cavity. Higher pressure can result in larger volume of intra-peritoneal space, which is easier for surgeons to perform the surgery. However, elevated intra-peritoneal pressure can lead to some adverse effects. The pressure-related complications included deep venous thrombosis, impaired cardiac contractility, impaired gas exchange and pulmonary embolism, etc. (1). In some studies of animal model, gasless laparoscopic surgery might reduce the risk of metastasis (2) and might reduce the stress-related biochemical status of the intraabdominal organs (3). Lung and chest compliance were influenced more in CO2 pneumoperitoneum than those in abdominal wall elevation method (4).
Another problem of incidental mass air leaks from the trocar sites can lead to immediate disappearance of operative fields. Surgeons have to re-inflate CO2 into the intra-peritoneal cavity to continue any procedure. Because maintaining adequate air volume within intra-abdominal cavity is quite essential for surgery, the size of trocar incision has to be tightly fit the size of trocar. The size of trocar also limits selection of instrument type and size.
In order to minimize the complications resulting from high intra-abdominal pressure and solve the problem of instrumentation, gasless laparoscopic surgery had been proposed in the past. The basic concept is using a device that can be fixed adequately on the operative table and a hook-type device that can lift the abdominal wall upwards. The lifting techniques and device can create a gasless operative filed but the volume of the operative field is very limited. Because of limited operative space, the method is not popular. In the study, we designed a highly folded device that can expand greatly after placement into peritoneal cavity and could act as a stent-like structure to obtaining an improved operative space in gasless laparoscopic surgery.
Methods
Device concept and in vitro simulation
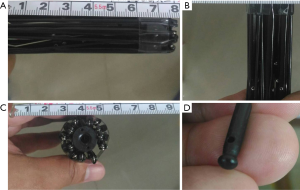
The basic structure is composed of multiple segments of stainless steel connected with joints. In order to minimize the size of the device, it was designed to be rod-like shape with highly folded structure (Figure 1A). The device is 8 cm in length with a diameter of 2 cm (Figure 1B,1C). The tip that would be anchored onto the abdominal wall is round with some friction force (Figure 1D). The reason that we designed the device to be a rod-like structure is assumed that it can be placed into the body cavity through the lifted abdominal incision, such as an umbilical incision. After placement into peritoneal cavity, a controller was used to pull, with which the structure can be expanded in one step. The expanded structure would resemble the appearance of a spider (Figure 2A,2B). There were four major joints in the device with repeated folding. The speed of the device expansion can be controlled manually by the operator. The overall appearance and working mechanism resembles the principles of umbrella in reverse direction.
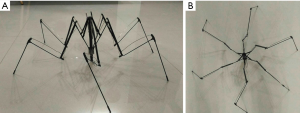
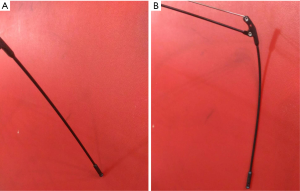
When the device expands, the distal tip will begin to touch the inner side of the abdominal wall (Figure 3A). With gradually increasing resistance during expansion, the most distal segment will be bent to form a doom-like appearance and the tip will be anchored in a certain location (Figure 3B). The tip of the device was designed to be blunt with rough surface to support the device in certain regions of the inner side of the abdominal wall.
Animal study using intra-abdominal stenting device
In the animal study, we tested the device in two 35-kg experimental pigs. Prior to beginning of the study, the pigs were prepared with an injection of intramuscular muscle relaxant. After induction of the anesthesia and intubation of an oral endotracheal tube, the pigs were adequately fixed on the operative tables. The whole course of anesthesia was maintained by inhalational anesthesia.
The basic instructions of the intra-abdominal stent were explained to the anticipated surgeons in detail. We confirmed that the surgeons could understand the instructions and could performed expansion of the stent in vitro.
The pigs were prepared in supine position. In order to test and compare the feasibility and efficacy of the stenting device, we let the surgeons to perform conventional laparoscopic surgery first by way of CO2 pneumoperitoneum and to proceed to the procedure using intra-abdominal stent.
After creation of CO2 pneumoperitoneum, the surgeons were allowed to perform simple procedures, such as splenectomy and cholecystectomy, which are easier procedures in tested pigs. After conventional laparoscopic surgery, we let the surgeons to try to use the umbilical incision to place the stent and then expand the stent device inside the peritoneal cavity of the pigs. After expansion of the intraabdominal stent, surgeons would perform simple procedures to check for the peritoneal cavity and observed the condition of the operative field as well as the abdominal wall that was anchored by the stent device. After the tests, we would allow the surgeons to extract the stent device outside to finish the animal study.
Results
The anticipated surgeons could understand the operation details of the intraabdominal stent within 5 minutes and could operate immediately after reading the instruction manual.
After adequate induction of general anesthesia, the planned procedures were placement of the intraabdominal stent and simulate simple laparoscopic procedures followed by withdraw of the intraabdominal stent to end the test.
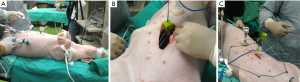
In the first step, a peri-umbilical incision was made (Figure 4A). The size of the incision was around 3 cm in diameter. The intraabdominal incision was used for direct visual inspection to ensure that there was no adhesion or diseased state. After confirming the peritoneal space to be safe for instrumentation, the abdominal wall was elevated by any available tool, such as a pair of hook (Figure 4B). The abdominal wall was elevated to the height that the device can be placed through the incision. Then the condition of the device and its surrounding environment was inspected by a rigid endoscope from another incision (Figure 4C). The device was expanded by the controller. Hence, the segments of the device could support the abdominal wall to form a tent-like working space (Figure 4D).

The space created by the intraabdominal stent was quite similar to CO2 pneumoperitoneum in the pressure of 15 mmHg (Figure 5A). Because the space was created by a semi-rigid mechanic structure, there would be no issues of air-leaks when we extended the incision. The condition was shown in Figure 5B. The space could be maintained by the structure without collapse. The comparison between the intraabdominal stent and the size of the abdomen was shown in Figure 5C. The stent size was slightly larger than the size of the abdomen.
The abdominal wall was intact during the 1-hour course of the simulation procedures. There was no acute injury in the abdominal wall, visceral organs, and the tip-anchored sites. In the tip-anchored sites, there was only mild ecchymosis without penetration of migration of the tip. During the whole course of the test, there was no acute complication.
Discussion
Along with development of laparoscopic surgery, CO2 pneumoperitoneum had become a standard method to create the operative space. However, CO2 pneumoperitoneum may result in some adverse reactions or complications, especially in some extensive and complex procedures and in the elderly population (2,5-7). Inflation of CO2 in the peritoneal cavity may cause compression of the venous system resulting in deep venous thrombosis and potentially lethal pulmonary embolism (8). During extensive dissection of the visceral organs, elevated air pressure may leak into the systemic circulation and contribute to the occurrence of air embolism (9). Cardiac tamponade and cardiac dysrhythmia are not uncommon in laparoscopic surgery (10-12). Cardiac contractility may be temporarily impaired by the compression of the intraabdominal air. Because CO2 is easier for absorption and it has inert chemical property, CO2 is a good choice than any other gas. But during prolonged procedure, absorption of CO2 can lead to acid-base imbalance as well as electrolyte imbalance, especially in the elderly population. Complete recovery may require protracted time in some conditions (1,13). Timely correction by increasing ventilator rate is mandatory during anesthesia to prevent complications (14-16). Tissue hypoperfusion resulting from CO2 pneumoperitoneum during laparoscopic surgery may aggravate the acid-base imbalance. Urine output can be decreased and the renal cortex perfusion may decrease to the extent of 60% soon after laparoscopic surgery, even in the pressure of less than 12 mmHg (17,18). The effects of laparoscopic surgery on the cerebral perfusion and oxygenation seemed to be controversial (19,20). Some studies indicated that there would be decrease in perioperative cerebral oxygenation while other studies showed that the difference is not significant (21,22).
The choice of inflated gas includes CO2, nitrous oxide, Helium and Argon. CO2 is the most favorable choice because its high solubility in the blood. However, CO2 is the only one choice that may irritate the peritoneum (23).
Since CO2 pneumoperitoneum has some disadvantages, some surgeons had tried to take use of gasless laparoscopic surgery in the hope to replace the role of conventional CO2 pneumoperitoneum (24,25). Soon after initial attempts, the issues became a debate (26). Gasless laparoscopy did not replace conventional laparoscopy using CO2 pneumoperitoneum. A major concern is that the operative space is narrow in gasless approach because the front abdominal wall was lifted upwards. The operative field is limited by this method. Therefore, most surgeons still use CO2 pneumoperitoneum during their routine operations. In order to improve the gasless approach, some researchers proposed different device concept (27-29).
The comparison of gaseous and gasless laparoscopic surgery is very limited. The only randomized trial was performed by the group of Goldberg et al. (30). In their study, it showed that gasless laparoscopy did improve ventilator function and can lower ventilator peak pressure during operation. In contrast to the advantages of pulmonary function, surgeon encountered more difficulty in performing surgery. In terms of wound pain and patient’s recovery, the difference is not significant. They also conclude that the device is limited and its function can’t fulfill the surgeon’s need.
Currently, the only product we can see in the market is Laparolift (Origin Medsystems, Menlo Park, CA, USA). With the device, the operative space can be created by lifting front abdominal wall by a metal plate connected with a device that can be anchored on the operative table. The maximal lifting force is around 13 kg, which is comparable to 15 mmHg CO2 pneumoperitoneum in creation of the operative space (31). The space, however, is an inverted V shape by lifting method, which is narrower. Some studies reported technical difficulty because the space is quite limited. In the study, 21.4% procedures had to be converted to CO2 pneumoperitoneum simply because the operative space was limited (30). In a clinical study performed by Johnson et al., 40% procedures had to be converted to CO2 pneumoperitoneum because the poor visibility during gasless laparoscopy (32). The result of these studies may demonstrate a fact that without suitable device, gasless laparoscopic surgery can not be a routine practice.
Recently, some studies showed that CO2 pneumoperitoneum may increase the possibility of peritoneal seeding of malignant cells and enhance tumor growth as well as local recurrence. The effects are minimized by gasless approach (33). Although it is a study of animal model, the results should be concerned.
In the setting of CO2 pneumoperitoneum, it can create a doom shape, which is better for both instrumentation and field-of-view. In contrast to the condition of currently available device, the lifting method can only create an inverted V shape. The issue of created space might be the key problem in current device. Inflation of gas into a closed space, such as the peritoneal cavity, can exert the force of expansion in all directions (Figure 6A). The volume of space may be related to the pressure. Pan-directional expanding force can generate a good operative space as in our routine laparoscopic surgery. When we tried to use the lifting device, the only reliable force is the lifting site. The resulting space is shown in Figure 6B, which is limited. When the target tissue or lesion is far from the lifting site, its surrounding space would be very small. If the procedure is complex and requires space, the likelihood of conversion to CO2 pneumoperitoneum increases. In order to overcome the problem of narrow space, we designed the device using the mechanism of stenting. The anchored site is marked as “*” in red in Figure 6C. Using multiple anchor sites to stabilize the device, it can generate the expanding force in multiple directions and resembled the structure of a tent with a dome (Figure 6C).
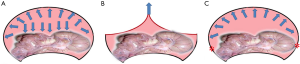
When the anchor sites are fixed adequately, the maximal supportive force of the structure is around 10 kg. The supportive force is less than the force exerted by the lifting device but the created space is far greater than that exerted by lifting device. Smaller force may result in minor pain and trauma in the abdominal wall.
The device has the advantages of larger operative space compared to that generated by the lifting device with even smaller supportive force. Only mild ecchymosis in the anchor sites in the abdominal wall can be seen in the proposed method. In the condition of CO2 pneumoperitoneum, we have to convert to open laparotomy if we need a large incision in complex procedures. The condition would be quite different in stenting method. Because extending incision will not cause air leaks, the stenting device can be still supportive and the working space will not collapse. If more trocar sites have to be created, surgeons can use nearly any site of the abdomen. The interference with the stent device is only minimal. Using large and conventional instrument, such as large and long needle holder or grasper, is much easier than the lifting methods. For gasless laparoscopy, stenting device may be a feasible alternative to lifting device.
There are some limitations of potential problems in the device. First, the size of the device should be adjusted according to the body size. The size selection is not a problem in the circumstances of using CO2 pneumoperitoneum and lifting device. We can only have a rough estimate of the size before operation and this should be based on experience in further clinical trial. Second, we generally have to use an endoscopy to ensure the security of its placement because there might be adhesion or any other pathologic state that may hinder placement of the device. Third, the complexity of the structure of the stenting device is related to the stability of the created space. In the study, we used a structure involving six segments. The supporting function is adequate. The comparison of the main three methods is listed in Table 1.
Table 1
| Characteristics | CO2 pneumoperitoneum | Lifting device | Stenting device |
|---|---|---|---|
| Mechanism | CO2 inflation | Pull-up force | Expanding stent |
| Mechanical trauma | None | More | Less |
| Chemical irritation | Yes | None | None |
| Created space | Large | Small | Large |
| Difficulty to create space | Easy | Easy | N/A* |
| Difficulty in operation | Easy | Difficult | Easy |
| Maintain working space | May collapse by air leaks | Yes | Yes |
| Impaired venous return | Yes | None | None |
| Impaired organ perfusion | Yes | None | None |
| Gas and electrolyte imbalance | Yes | None | None |
| How to adjust space | Adjust air pressure | Increase lifting force | Choose larger size |
| Incision size | Fit to trocar size | Adjustable | Adjustable |
*, The user experience could not be established by the animal study.
In the preliminary animal study, the feasibility and plausibility can be confirmed. However, clinical study has to be carried out to test for its safety and efficacy in both patients and surgeons.
Acknowledgments
Funding: The authors would like to thank the National Taipei University of Technology and Mackay Memorial Hospital, Taiwan for financially supporting this research under Contract No. MMH-TT-10501.
Footnote
Conflicts of Interest: CHC serves as an Associate Editor-in-Chief of Health Technology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee and in compliance with the international guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rademaker BM, Meyer DW, Bannenberg JJ, et al. Laparoscopy without pneumoperitoneum. Effects of abdominal wall retraction versus carbon dioxide insufflation on hemodynamics and gas exchange in pigs. Surg Endosc 1995;9:797-801. [PubMed]
- Ishida H, Hashimoto D, Takeuchi I, et al. Liver metastases are less established after gasless laparoscopy than after carbon dioxide pneumoperitoneum and laparotomy in a mouse model. Surg Endosc 2002;16:193-6. [Crossref] [PubMed]
- Guven S, Muci E, Unsal MA, et al. The effects of carbon dioxide pneumoperitoneum on ovarian blood flow, oxidative stress markers, and morphology during laparoscopy: a rabbit model. Fertil Steril 2010;93:1327-32. [Crossref] [PubMed]
- Casati A, Valentini G, Ferrari S, et al. Cardiorespiratory changes during gynaecological laparoscopy by abdominal wall elevation: comparison with carbon dioxide pneumoperitoneum. Br J Anaesth 1997;78:51-4. [Crossref] [PubMed]
- D'Ercole C, Cravello L, Guyon F, et al. Gasless laparoscopic gynecologic surgery. Eur J Obstet Gynecol Reprod Biol 1996;66:137-9. [Crossref] [PubMed]
- Li B, Hao J, Gao X, et al. Gynecological procedures under gasless laparoscopy. Chin Med J (Engl) 2001;114:514-6. [PubMed]
- Lukban JC, Jaeger J, Hammond KC, et al. Gasless versus conventional laparoscopy. N J Med 2000;97:29-34. [PubMed]
- Inderbitzin DT, Opitz I, Giger U, et al. Incidence of clinical pulmonary embolism after laparoscopic surgery. Br J Surg 2007;94:599-603. [Crossref] [PubMed]
- Greville AC, Clements EA, Erwin DC, et al. Pulmonary air embolism during laparoscopic laser cholecystectomy. Anaesthesia 1991;46:113-4. [Crossref] [PubMed]
- Farlo J, Thawgathurai D, Mikhail M, et al. Cardiac tamponade during laparoscopic Nissen fundoplication. Eur J Anaesthesiol 1998;15:246-7. [Crossref] [PubMed]
- Talamini MA, Mendoza-Sagaon M, Gitzelmann CA, et al. Increased mediastinal pressure and decreased cardiac output during laparoscopic Nissen fundoplication. Surgery 1997;122:345-52; discussion 52-3. [Crossref] [PubMed]
- Firoozmand E, Ritter M, Cohen R, et al. Ventricular laceration and cardiac tamponade during laparoscopic Nissen fundoplication. Surg Laparosc Endosc 1996;6:394-7. [Crossref] [PubMed]
- Hsing CH, Hseu SS, Tsai SK, et al. The physiological effect of CO2 pneumoperitoneum in pediatric laparoscopy. Acta Anaesthesiol Sin 1995;33:1-6. [PubMed]
- Lee KC, Kim JY, Kwak HJ, et al. The effect of heating insufflation gas on acid-base alterations and core temperature during laparoscopic major abdominal surgery. Korean J Anesthesiol 2011;61:275-80. [Crossref] [PubMed]
- Garg R, Punj J, Pandey R, et al. Delayed recovery due to exaggerated acid, base and electrolyte imbalance in prolonged laparoscopic repair of diaphragmatic hernia. Saudi J Anaesth 2011;5:79-81. [Crossref] [PubMed]
- Kwak HJ, Jo YY, Lee KC, et al. Acid-base alterations during laparoscopic abdominal surgery: a comparison with laparotomy. Br J Anaesth 2010;105:442-7. [Crossref] [PubMed]
- Iwase K, Takenaka H, Ishizaka T, et al. Serial changes in renal function during laparoscopic cholecystectomy. Eur Surg Res 1993;25:203-12. [Crossref] [PubMed]
- Chiu AW, Chang LS, Birkett DH, et al. Changes in urinary output and electrolytes during gaseous and gasless laparoscopy. Urol Res 1996;24:361-6. [Crossref] [PubMed]
- Tuna AT, Akkoyun I, Darcin S, et al. Effects of carbon dioxide insufflation on regional cerebral oxygenation during laparoscopic surgery in children: a prospective study. Rev Bras Anestesiol 2016;66:249-53. [Crossref] [PubMed]
- Mousa WF, Mowafi HA, Al-Metwalli RR, et al. Preoperative mannitol infusion improves perioperative cerebral oxygen saturation and enhances postoperative recovery after laparoscopic cholecystectomy. Saudi Med J 2015;36:1199-204. [Crossref] [PubMed]
- Mynbaev OA, Gerntke I, Tinelli A, et al. Letter to the Editor: The Effect of Ventilation Strategy on Arterial and Cerebral Oxygenation During Laparoscopic Bariatric Surgery. Obes Surg 2016;26:1599-600.
- Kemerci PU, Demir A, Aydinli B, et al. 10 cm H2O PEEP application in laparoscopic surgery and cerebral oxygenation: a comparative study with INVOS and FORESIGHT. Surg Endosc 2016;30:971-8. [Crossref] [PubMed]
- Liu Y, Hou QX. Effect of carbon dioxide pneumoperitoneum during laparoscopic surgery on morphology of peritoneum. Zhonghua Yi Xue Za Zhi 2006;86:164-6. [PubMed]
- . Gasless laparoscopy eliminates complications caused by pneumoperitoneum. Minim Invasive Surg Nurs 1994;8:2-6. [PubMed]
- Smith RS, Fry WR, Tsoi EK, et al. Gasless laparoscopy and conventional instruments. The next phase of minimally invasive surgery. Arch Surg 1993;128:1102-7. [Crossref] [PubMed]
- Hill DJ, Maher PJ, Wood EC. Gasless laparoscopy--useless or useful? J Am Assoc Gynecol Laparosc 1994;1:265-8. [Crossref] [PubMed]
- Adachi S, Furukawa N, Morimura A, et al. Gynecological laparoscopic surgery by gasless laparoscopy by using laparolift. Nihon Sanka Fujinka Gakkai Zasshi 1995;47:961-3. [PubMed]
- Kenyon T, Lenker M, Underwood K. Gasless laparoscopy with mechanical peritoneal distention. Minim Invasive Surg Nurs 1994;8:62-7. [PubMed]
- Chin AK, Eaton J, Tsoi EK, et al. Gasless laparoscopy using a planar lifting technique. J Am Coll Surg 1994;178:401-3. [PubMed]
- Goldberg JM, Maurer WG. A randomized comparison of gasless laparoscopy and CO2 pneumoperitoneum. Obstet Gynecol 1997;90:416-20. [Crossref] [PubMed]
- Chin AK, Moll FH, McColl MB, et al. Mechanical peritoneal retraction as a replacement for carbon dioxide pneumoperitoneum. J Am Assoc Gynecol Laparosc 1993;1:62-6. [Crossref] [PubMed]
- Johnson PL, Sibert KS. Laparoscopy. Gasless vs. CO2 pneumoperitoneum. J Reprod Med 1997;42:255-9. [PubMed]
- Bouvy ND, Marquet RL, Jeekel H, et al. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg 1996;224:694-700; discussion -1.
Cite this article as: Chen CH, Chang H, Liu TP, Liu HC, Chen CH. An intraabdominal stent system for gasless laparoscopic surgery in animal model: potential benefits and limitations. Health Technol 2017;1:4.


